For this 2017 AIS research project, the target species included larval Asian Carps (Black, Bighead, Silver, and Grass Carps) and new aquatic invasive species. To best catch these novel non-native species, the USFWS used a combination of bongo nets and quatrefoil light traps at five hotspot locations (Hayer 2017).
 AIS Research at Chicago Harbor, IL USFWS. 2017. |
 AIS Research in Lake Michigan USFWS. 2017. |
Bongo Nets
Bongo nets are an active gear type for surveying for ichthyoplankton (eggs and larvae of fish). A 500-µm-mesh set of paired conical bongo nets (0.6-m-diameter mouth) equipped with mechanical flow meters (General Oceanics Model 2030R) were placed in a fixed position along the boat’s side to sample the top three feet of the water column. The bongo nets were mounted when sampling in deep offshore areas, open water, or obstruction free areas. These nets remained in a fixed position while the boat was stationary for five minutes. Bongo nets captured more eggs and yolk-stage larvae compared to light traps. The capture rate of yolk-stage larvae is increased since they are less mobile due to limited fin development (Neal et al. 2012). This research project taught us that the efficiency of capturing larval fish changed depending on the gear type throughout the spawning season.
 Bongo Nets Locked for Five Minutes. USFWS. 2017. |
 Removal of Bongo Nets from Research Site. USFWS. 2017. |
 Bongo Nets Being Deployed in Green Bay, WI. USFWS. 2017. |
Quatrefoil Light Traps
Quatrefoil light traps (0.3-m-diameter, 0.25-m-tall, gap width 5 mm) are a passive gear type equipped with waterproof flashlights and light diffusers. At dusk, these traps were deployed near shallow areas or backwater habitats (open to the channel at one end). Within the sampling areas, these traps were set in water depths ranging from 1 – 5 meters and distributed uniformly in both nearshore and offshore areas. Light traps are placed in shallow habitats since they are targeting larval fish that are beginning to develop fins and tend to swim in less pelagic areas to obtain food and avoid predation.
Some physical factors that affect the capture of larval species are the development of visual systems, swimming abilities, behavioral responses to light, water clarity, and current velocity (Milicich et al. 1992 & Thorrold 1992). While bongo nets captured more eggs and yolk-stage larvae, light traps collected larger individuals and more species. Larval fish were collected in a 2-L bottle threaded to the bottom of the trap since larval fish are attracted to light and swim towards the light trap’s funnel gap (Brogan 1994). Usually, quatrefoil light traps are soaked overnight and picked up the following morning. The specimens caught within the light traps were placed in bottles containing 95% non-denatured ethyl alcohol and labeled with the gear type, date of collection, total specimen count, length category of larvae, and sampling site ID.
 Deploying Light Traps at Dusk. USFWS. 2017. |
 Deploying Light Traps at Dusk. USFWS. 2017. |
 Quatrefoil light traps (0.3-m-diameter, 0.25-m-tall, gap width 5 mm) with waterproof flashlights and light diffusers. USFWS. 2017. |
 Deploying Light Traps at Dusk. USFWS. 2017. |
Laboratory Research
To examine light trap and bongo net samples, the larvae and fish eggs were first removed from the rest of the debris and placed in an ethyl alcohol solution vial. Once the specimens are placed in a Petrie dish with an ethyl alcohol solution, there are certain steps you will follow to properly identify these larval fish species.
- Using a dissecting microscope (Nikon SMZ-1270) equipped with a camera
(Nikon NIS-Elements software), biological technicians first adjust the scope light
so that it comes from one side to help illuminate the myomeres. Without angled bright light, it is hard to detect myomeres with sufficient accuracy. Then, technicians begin measurements. - After measuring the larvae from their mouth to the tip of their tail (Shaffer 2016),
then the myomeres (muscle segments) are counted. Counting myomeres are the most important morphometric structure in identifying larval fish. - Muscles are separated into two categories: preanal and postanal counts.
- Preanal myomeres are counted from the nape to the imaginary vertical line at the most posterior anus.
- The postanal myomeres are counted from the imagery vertical line at the most posterior anus to the edge of the caudal (tail) fin where they become thin and compressed and form the urostyle.
- Counting myomeres can be imprecise, so your count is probably accurate to within a few myomeres.
- Using this imagery, larval fish specimens were identified using a dichotomous key to classify larvae to the lowest possible taxonomic level (Auer 1982).
- Dr. Auer’s Dichotomous Key
 Larval Fish Samples in a 95% Ethyl Alcohol Solution Vials. USFWS/Adam Dziewa. 2018. |
 Larval Fish Samples in a 95% Ethyl Alcohol Solution Petrie Dish. USFWS/Adam Dziewa. 2018. |
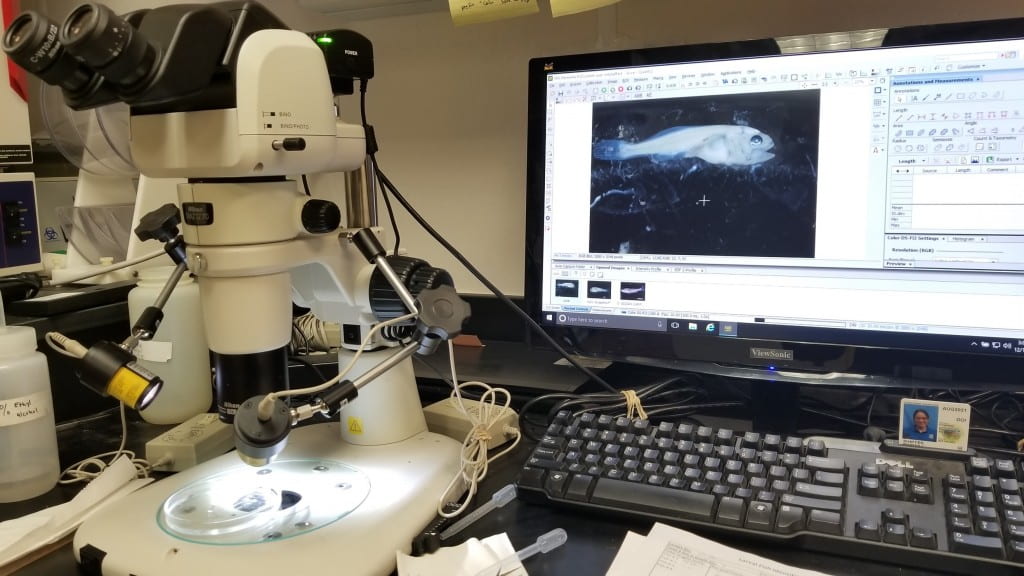 Dissecting Microscope (Nikon SMZ-1270) Equipped with a Camera (Nikon NIS-Elements software). USFWS/Adam Dziewa. 2018. |
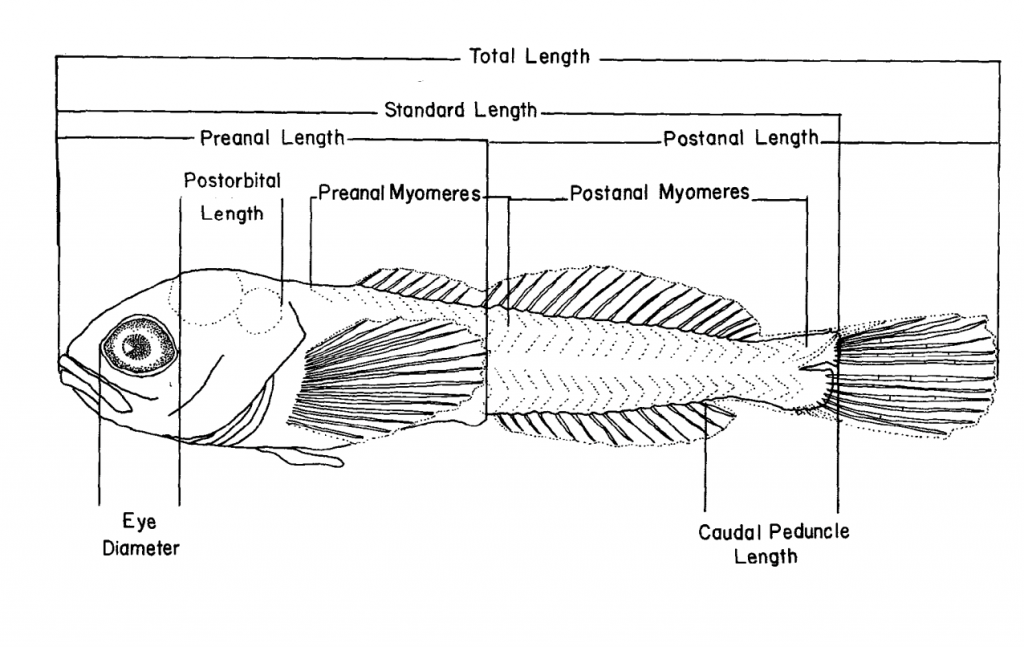
Illustration of a Teleost Larvae’s Morphology (Auer 1982). *Click on the picture to access the web resource.*
